Future Materials – PEF, Hype or Hero?
PEF - What is it?
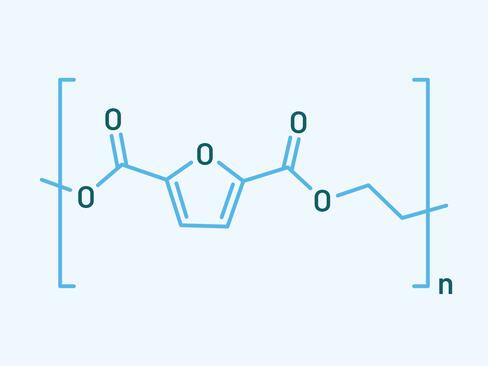
First, a little chemistry to start up the brain. Polyethylene furanoate (PEF) is mainly synthesized from 2,5-furandicarboxylic acid (FDCA) and ethylene glycol. FDCA can be sourced from biogenic carbohydrates making it an important building block for sustainable chemistry. With this mainly bio-based feedstock as an asset, companies like Avantium in the Netherlands set out to substitute the incumbent aromatic polyesters like PET.

Properties and Outlook
Now, what about the properties? As the chemical structures of PEF and PET are very similar, so are the technical properties. But PEF exhibits an even better gas barrier than PET. Additionally, the melting point is lower whereas the glass transition temperature is higher. This saves energy in the production process, but gives PEF an advantage in regards to heat related applications like hot beverages. Naturally, PEF is still more expensive than PET, as its development is in an early stage, but the great head start in environmental impact categories makes it a promising candidate for PET replacement.
A recent study by Avantium on PEF bottles showed, that already in this early stage, a significant reduction of 35% greenhouse gas (GHG) emissions is achieved compared to PET. With optimization of production process and FDCA chemistry there is way more to come.
PEF Natural Fiber Composite Materials
As we are the cellulose guys, we surely looked into PEF natural fiber composites and found… nothing. It seems, the new guy among the bioplastics was not yet seriously investigated for natural fiber composites applications. Looking on the technical side, a melting point of little over 200 °C will be challenging for cellulose composite production. But with refined compounding technology it might be feasible, the coming years will show. If you want to investigate, you know where to call.
To answer the header's question, there is quite a hype ongoing in the bioplastics industry. However, to make PEF also a hero, some major technical and commercial obstacles need to be overcome. At least, the heroic potential is there.









